
Easily manage patient enrollment.
Improve data accuracy and performance by managing everything from pre-screening and referral management to visits and financials in one easy-to-use clinical trial management system that scales to fit your site’s needs.
How StudyTeam Helps Sites
Recruitment
The suite you need to become a preferred site
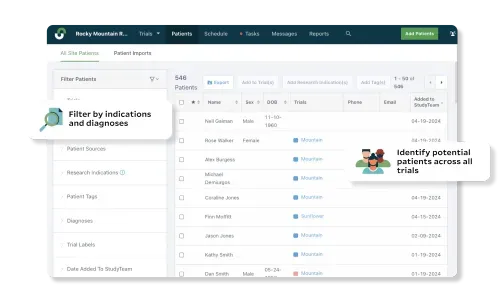
Leverage all of your staff’s efforts during recruitment to build detailed, digital patient databases and give your next trial a head-start. Feel confident about hitting enrollment goals with a list of potential candidates filterable by pre-screened I/E criteria.
Referral Vendor Management
Manage your referrals centrally
Stop wasting time logging into multiple systems to evaluate all of your recruitment efforts. Eliminate duplicate data entry and lost referrals with digital integration and centralized referral management.

Direct Scheduling
Visit-Ready referrals scheduled for your site
Streamline your recruitment efforts with Direct Scheduling for Care Access Future of Medicine referrals. We partner with Care Access to take care of the scheduling, calendaring, and reminder work for you.

Why StudyTeam® for Sites?
Consolidate staff efforts and maximize patient selection for recruitment
Pre-screen patients and automatically create a patient database with filterable I/E criteria to help you enroll studies across multiple trials more efficiently.
Why Pre-screening Data Matters in Clinical Trials That Are on Track with Enrollment
Facilitate sponsor engagement to attract more trials
Keep sponsors updated on your trial’s status in real-time while cutting down status calls, emails, and manual log submissions with built-in, automatically de-identified sponsor reporting.
Percentage of AstraZeneca oncology sites that choose to use StudyTeam for Sites on subsequent studies
100%A critical tool for research sites
“StudyTeam has been a game-changer for our site network. It takes hours-long processes that our coordinators were required to do by the sponsors and compresses them to minutes – giving us more time to work with and educate patients. StudyTeam also helps us recruit and enroll qualified patients more effectively. Our entire team can work together seamlessly in the system increasing our capacity to enroll the right patients in the right trials quickly.”
Rebecca Goldfaden, PharmD, CCRP Vice President of Clinical Operations East Coast Institute for ResearchFAQs
What is Care Access Future of Medicine?
Future of Medicine is an always-on, IRB-approved, and study-agnostic community health screening program, continually growing its population of highly-qualified, pre-screened members. Learn more here.
What is Care Access?
Care Access is a team composed of seasoned professionals across different industries who have come together, in collaboration with clinical research experts, to tackle some of the toughest challenges in clinical trial delivery.
Together, Care Access develops new innovative systems that help doctors and healthcare professionals extend the reach of clinical research and its benefits to more communities than ever before, and accelerate the development of new cures and treatments.
How many users can I have at my site?
You get a software application designed for the workflow of clinical research coordinators, recruitment coordinators, and site directors. You get rich patient recruitment and patient enrollment reports, an intuitive patient recruitment tracker, and a database for your research patients. You also get industry-leading support from OneStudyTeam's Customer Success team, ready to answer questions and provide guidance for using the software.
What do I get when I activate StudyTeam for my site?
You get a software application designed for the workflow of clinical research coordinators, recruitment coordinators, and site directors. You get rich patient recruitment and patient enrollment reports, an intuitive patient recruitment tracker, and a database for your research patients. You also get industry-leading support from OneStudyTeam's Customer Success team, ready to answer questions and provide guidance for using the software.
How does StudyTeam streamline communication with sponsors?
When both you and your sponsor are using StudyTeam, you can share important information about patient recruitment and pre-screening with your sponsor easily and automatically. This cuts out the need to email, fax, or call in recruitment updates, thus saving you valuable time that you can devote to caring for patients.
How many users can I have at my site?
An unlimited number of users at your site can access StudyTeam. Each user can log into your StudyTeam account and simultaneously view the same information, which makes for faster, more collaborative patient recruitment and patient enrollment management.
In which countries is StudyTeam for Sites available?
StudyTeam is currently available in 105 countries and counting. We are actively expanding to new regions — please contact us to get our most up-to-date country map.
What languages is StudyTeam for Sites available in?
StudyTeam for Sites can be viewed in the following languages:Japanese, Korean, German, Chinese, Spanish
Coming this year: Portuguese, French, Turkish, Czech, Italian, Greek, Russian, Bulgarian, Polish, Hungarian
Contact us if you don't see your country or preferred language.









