Our Blog
Consejos para el Reclutamiento y Educación de Pacientes de un Coordinador de Investigación Clínica de REIMED en Sudáfrica
October 1, 2024Al coordinador de investigación clínica Luke Francke le apasiona conocer gente nueva y ayudar a la comunidad desfavorecida de Johannesburgo (Sudáfrica).

Mejorando el Cuidado de los Pacientes: 6 Estrategias para los Centros de Investigación
October 1, 2024Mejorar el cuidado de los pacientes en los centros de investigación clíinica apoya el éxito de los ensayos clínicos.

6 Ways to Reduce Protocol Deviations in Clinical Trials with StudyTeam Technology
October 1, 2024It’s paramount that site teams are empowered to adhere to protocols. Read on to learn how StudyTeam can help limit protocol deviations in clinical trials.
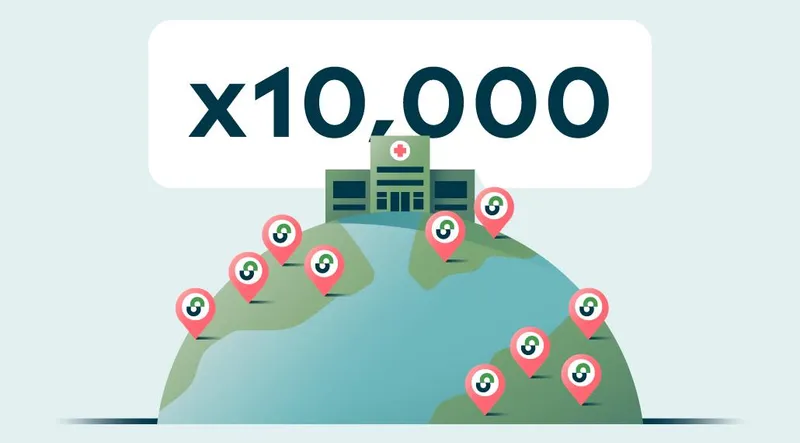
OneStudyTeam Expands Global Reach to More than 10,000 Research Sites
September 24, 2024OneStudyTeam announced that StudyTeam for Sites clinical trial software is now being used by more than 10,000 clinical research sites globally.

Improving Patient Care: 6 Strategies for Research Sites
September 9, 2024Better patient care at research sites supports the success of clinical trials. Here are 6 strategies sites can use for improving patient care.

How Can Your Site Stand Out On a Clinical Trial Site Feasibility Questionnaire? Track These 3 Metrics.
August 20, 2024Here are 3 powerful site feasibility metrics to track for questionnaires, plus where to find them if you use StudyTeam for Sites.
